Abstract
Background: Ibrutinib (IBR) and venetoclax (VEN) combination is a highly effective therapy for patients (pts) with CLL (Jain, NEJM 2019; Wierda, ASH 2020; Kater, EHA 2021). We previously reported results of the first-line cohort of a phase II trial of combined IBR and VEN for high-risk pts with CLL (Jain, NEJM 2019; Jain, JAMA Oncology 2021). Here we report updated data for these pts with focus on MRD.
Methods: Pts with previously untreated CLL meeting IWCLL treatment criteria were enrolled. All pts had at least one high-risk feature: del(17p), mutated TP53, del(11q), unmutated IGHV, or age ≥65 years (yrs). Pts received IBR 420 mg daily for 3 cycles followed by addition of VEN (weekly dose-escalation to 400mg daily). Combined therapy was given for 24 cycles (28 days/cycle). Pts with bone marrow (BM) undetectable MRD (U-MRD) (flow cytometry; sensitivity 10 -4) at 24 cycles of combined therapy discontinued both VEN and IBR; MRD+ pts continued IBR. A trial amendment allowed an additional 12 cycles of combined VEN and ibrutinib for pts who remained BM MRD+ after Cycle 24. Response assessments were performed using BM and CT imaging studies (2008 IWCLL criteria). U-MRD was defined as <0.01%; low MRD+ 0.01% to <1%; high MRD+ ≥1%. Progression-free survival (PFS) was assessed as the time from the start of study drug to CLL progression, Richter transformation, or death from any cause. Blood MRD was monitored every 6 months in pts off treatment or on ibrutinib monotherapy beyond 24 cycles of combined treatment.
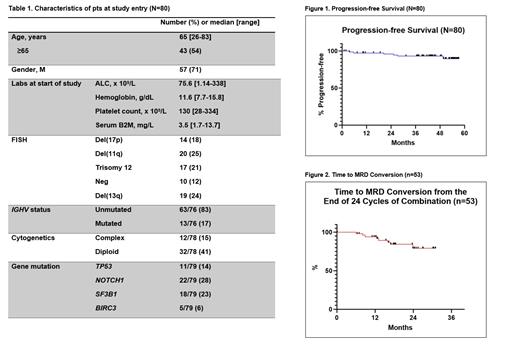
Results: A total of 80 pts were enrolled. Baseline characteristics are shown in Table 1. The median follow-up was 44.1 months.
Five pts came off study during 1 st 3 cycles of IBR monotherapy; 75 pts initiated VEN. We previously reported that after 12 cycles of the combination, 45/80 (56%) achieved BM U-MRD remission; 24/80 (30%) were BM MRD-positive (low MRD+, n=19; high MRD+, n=5). After 24 cycles of the combination, 53/80 (66%) achieved BM U-MRD remission; 14/80 (17%) were BM MRD+ (low MRD+, n=13; high MRD+, n=1). Overall, 60/80 (75%) achieved BM U-MRD as the best response. Updated PFS is provided in Figure 1.
Of the 53 pts who were BM U-MRD at the end of cycle 24 of the combination, 52 pts had a subsequent blood MRD assessment done in follow-up (1 missed due to COVID-19); 51/53 discontinued all therapy, 2 pts continued IBR per treatment physician discretion. With a median time of 18.4 months post Cycle 24, 8 pts had recurrence of blood MRD (defined as MRD ≥ 0.01% in 2 consecutive visits) in follow-up with 1 pt with CLL progression. The sole pt with CLL progression had mutated IGHV with del(11q) and NOTCH1 mutation. The pt had delayed achievement of BM U-MRD with the pt achieving U-MRD for the first time at the end of Cycle 24 of combined therapy. She was noted to have disease progression 22 months off therapy; BTK or PLCG2 mutation were not detected and the patient is currently in clinical remission on acalabrutinib. The time to MRD conversion for these 53 pts is shown in Figure 2.
There were 14 pts who were BM MRD+ at the end of cycle 24 of the combination (low MRD+, n=13; high MRD+, n=1). The only pt with high-MRD+ at end of cycle 24 was noted to have Richter transformation at that time. The remaining 13 pts (all low MRD+ in BM, range 0.01-0.56%) continued IBR monotherapy. With a recent trial amendment, MRD+ pts after Cycle 24 could get 12 additional cycles of venetoclax; 9/13 pts have resumed VEN. 6/9 pts have achieved U-MRD remission.
2 pts had Richter transformation and 3 pts have died (Jain, JAMA Oncology 2021).
Conclusions: We report long term follow-up of combined IBR and VEN in first-line CLL. Remissions were durable with some pts having recurrence of blood MRD in follow-up, which may be an early indicator of relapse. In a small subset of the pts with BM MRD+ disease at 24 cycles of combined therapy, additional VEN appears to lead to U-MRD remission in majority of the pts. Whether this will lead to improved long-term PFS remains to be determined.
Jain: TG Therapeutics: Honoraria; Beigene: Honoraria; Janssen: Honoraria; Fate Therapeutics: Research Funding; Aprea Therapeutics: Research Funding; Precision Biosciences: Honoraria, Research Funding; Incyte: Research Funding; Adaptive Biotechnologies: Honoraria, Research Funding; Cellectis: Honoraria, Research Funding; ADC Therapeutics: Honoraria, Research Funding; Servier: Honoraria, Research Funding; Pfizer: Research Funding; Bristol Myers Squibb: Honoraria, Research Funding; AstraZeneca: Honoraria, Research Funding; Genentech: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; Pharmacyclics: Research Funding. Thompson: AbbVie: Other: Institution: Advisory/Consultancy, Honoraria, Research Grant/Funding; Amgen: Other: Institution: Honoraria, Research Grant/Funding; Genentech: Other: Institution: Advisory/Consultancy, Honoraria, Research Grant/Funding; Adaptive Biotechnologies: Other: Institution: Advisory/Consultancy, Honoraria, Research Grant/Funding, Expert Testimony; Pharmacyclics: Other: Institution: Advisory/Consultancy, Honoraria, Research Grant/Funding; Janssen: Consultancy, Honoraria; Gilead: Other: Institution: Advisory/Consultancy, Honoraria. Ferrajoli: BeiGene: Other: Advisory Board, Research Funding; Janssen: Other: Advisory Board ; AstraZeneca: Other: Advisory Board, Research Funding. Burger: Novartis: Other: Travel/Accommodations/Expenses, Speakers Bureau; TG Therapeutics: Other: Travel/Accommodations/Expenses, Research Funding, Speakers Bureau; Janssen: Consultancy, Other: Travel/Accommodations/Expenses, Speakers Bureau; Beigene: Research Funding, Speakers Bureau; Pharmacyclics LLC: Consultancy, Other: Travel/Accommodations/Expenses, Research Funding, Speakers Bureau; Gilead: Consultancy, Other: Travel/Accommodations/Expenses, Research Funding, Speakers Bureau; AstraZeneca: Consultancy. Borthakur: GSK: Consultancy; ArgenX: Membership on an entity's Board of Directors or advisory committees; University of Texas MD Anderson Cancer Center: Current Employment; Protagonist: Consultancy; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Astex: Research Funding; Ryvu: Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees. Takahashi: Symbio Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy; Celgene/BMS: Consultancy; GSK: Consultancy. Sasaki: Daiichi-Sankyo: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Research Funding. Kadia: Cellonkos: Other; Aglos: Consultancy; Dalichi Sankyo: Consultancy; AbbVie: Consultancy, Other: Grant/research support; BMS: Other: Grant/research support; Amgen: Other: Grant/research support; Cure: Speakers Bureau; Jazz: Consultancy; Genentech: Consultancy, Other: Grant/research support; Liberum: Consultancy; Novartis: Consultancy; Pfizer: Consultancy, Other; Pulmotech: Other; Sanofi-Aventis: Consultancy; AstraZeneca: Other; Astellas: Other; Genfleet: Other; Ascentage: Other. Konopleva: Sanofi: Other: grant support, Research Funding; Cellectis: Other: grant support; Calithera: Other: grant support, Research Funding; KisoJi: Research Funding; Agios: Other: grant support, Research Funding; Ascentage: Other: grant support, Research Funding; AbbVie: Consultancy, Honoraria, Other: Grant Support, Research Funding; Ablynx: Other: grant support, Research Funding; Stemline Therapeutics: Research Funding; Eli Lilly: Patents & Royalties: intellectual property rights, Research Funding; AstraZeneca: Other: grant support, Research Funding; Rafael Pharmaceuticals: Other: grant support, Research Funding; Genentech: Consultancy, Honoraria, Other: grant support, Research Funding; F. Hoffmann-La Roche: Consultancy, Honoraria, Other: grant support; Forty Seven: Other: grant support, Research Funding; Novartis: Other: research funding pending, Patents & Royalties: intellectual property rights; Reata Pharmaceuticals: Current holder of stock options in a privately-held company, Patents & Royalties: intellectual property rights. Alvarado: BerGenBio: Research Funding; Jazz Pharmaceuticals: Research Funding; Astex Pharmaceuticals: Research Funding; Sun Pharma: Consultancy, Research Funding; MEI Pharma: Research Funding; FibroGen: Research Funding; Daiichi-Sankyo: Research Funding; CytomX Therapeutics: Consultancy. Yilmaz: Pfizer: Research Funding; Daiichi-Sankyo: Research Funding. DiNardo: Notable Labs: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria; Takeda: Honoraria; Celgene, a Bristol Myers Squibb company: Honoraria, Research Funding; Forma: Honoraria, Research Funding; AbbVie: Consultancy, Research Funding; GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Honoraria, Research Funding; ImmuneOnc: Honoraria, Research Funding; Agios/Servier: Consultancy, Honoraria, Research Funding; Foghorn: Honoraria, Research Funding. Bose: Kartos Therapeutics: Honoraria, Research Funding; Sierra Oncology: Honoraria; Novartis: Honoraria; Constellation Pharmaceuticals: Research Funding; NS Pharma: Research Funding; Celgene Corporation: Honoraria, Research Funding; Blueprint Medicines: Honoraria, Research Funding; Pfizer: Research Funding; Promedior: Research Funding; Astellas: Research Funding; Incyte Corporation: Honoraria, Research Funding; BMS: Honoraria, Research Funding; CTI BioPharma: Honoraria, Research Funding. Pemmaraju: Blueprint Medicines: Consultancy; LFB Biotechnologies: Consultancy; Novartis Pharmaceuticals: Consultancy, Other: Research Support, Research Funding; ASCO Leukemia Advisory Panel: Membership on an entity's Board of Directors or advisory committees; Dan's House of Hope: Membership on an entity's Board of Directors or advisory committees; Roche Diagnostics: Consultancy; MustangBio: Consultancy, Other; Affymetrix: Consultancy, Research Funding; Samus: Other, Research Funding; ImmunoGen, Inc: Consultancy; ASH Communications Committee: Membership on an entity's Board of Directors or advisory committees; Aptitude Health: Consultancy; Plexxicon: Other, Research Funding; Springer Science + Business Media: Other; Protagonist Therapeutics, Inc.: Consultancy; HemOnc Times/Oncology Times: Membership on an entity's Board of Directors or advisory committees; Clearview Healthcare Partners: Consultancy; Abbvie Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding; CareDx, Inc.: Consultancy; Sager Strong Foundation: Other; Daiichi Sankyo, Inc.: Other, Research Funding; Incyte: Consultancy; Stemline Therapeutics, Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding; Bristol-Myers Squibb Co.: Consultancy; DAVA Oncology: Consultancy; Pacylex Pharmaceuticals: Consultancy; Celgene Corporation: Consultancy; Cellectis S.A. ADR: Other, Research Funding. Jabbour: Amgen, AbbVie, Spectrum, BMS, Takeda, Pfizer, Adaptive, Genentech: Research Funding. Wang: Stemline Therapeutics: Honoraria. Kantarjian: Taiho Pharmaceutical Canada: Honoraria; Precision Biosciences: Honoraria; Immunogen: Research Funding; Daiichi-Sankyo: Research Funding; Jazz: Research Funding; BMS: Research Funding; AbbVie: Honoraria, Research Funding; Pfizer: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; NOVA Research: Honoraria; KAHR Medical Ltd: Honoraria; Ipsen Pharmaceuticals: Honoraria; Astra Zeneca: Honoraria; Astellas Health: Honoraria; Aptitude Health: Honoraria; Amgen: Honoraria, Research Funding; Ascentage: Research Funding. Wierda: Juno Therapeutics: Research Funding; AstraZeneca: Research Funding; Xencor: Research Funding; Janssen: Research Funding; Loxo Oncology, Inc.: Research Funding; Cyclacel: Research Funding; Oncternal Therapeutics, Inc.: Research Funding; Miragen: Research Funding; KITE Pharma: Research Funding; Sunesis: Research Funding; Gilead Sciences: Research Funding; Acerta Pharma Inc.: Research Funding; Pharmacyclics LLC, an AbbVie Company: Research Funding; Karyopharm: Research Funding; Genentech: Research Funding; GSK/Novartis: Research Funding; Genzyme Corporation: Consultancy; AbbVie: Research Funding.
The combination of ibrutinib and venetoclax is not FDA approved

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal